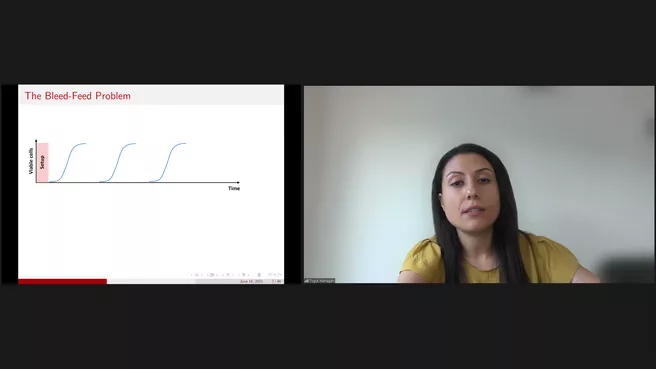
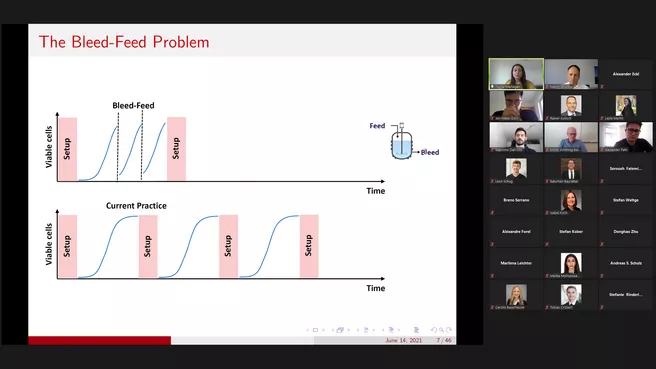
In the first part of the seminar, we will focus on operational decisions to improve biomanufacturing efficiency. Bleed-feed is a novel technology that allows biomanufacturers to skip intermediary bioreactor setups. However, the specific time at which bleed-feed is performed is critical for success. The process is stringently regulated, and its implementation involves unique trade-offs in operational decision-making. Our analysis formalizes the operational challenges related to bleed-feed decisions, and our results inform biomanufacturers and policymakers on the potential impact of this technology on current practice. We develop a finite-horizon, discrete-time Markov decision process, and analyze the structural characteristics of optimal bleed-feed policies. The research has been conducted in close collaboration with Merck (MSD) in the Netherlands. Real-world implementation at MSD resulted in 85% improvement in the batch yield (A brief video on this project is available at https://www.youtube.com/watch?v=79B7OBuvRkY&feature=youtu.be).
In the second part of the seminar, we will focus on strategic R&D decisions and analyze innovative pricing and payment schemes suggested for rare diseases. There are more than 7,000 known rare diseases, but pharmaceutical manufacturers developed treatments for only 500 of them. To improve the availability and accessibility of treatments for rare diseases, governments have introduced several programs including subsidies, innovative pricing, and outcome-based payment schemes. However, there is no consensus as to whether these programs would improve patient access to these treatments.
Inspired by ongoing pilot programs, we consider an exogenous pricing strategy that delegates the pricing decision to an independent consortium. We also examine an outcome-based ``No Pay No Cure" payment scheme, which offers the drug for free if it is not efficacious. We formulate a 4-stage Stackelberg game to determine whether it is optimal to subsidize the pharmaceutical manufacturer, the patients, or both under different pricing and payment schemes. Our analysis reveals that it is optimal to offer subsidies to pharmaceutical manufacturers. In addition, we show that delegating the pricing decision to an independent consortium can improve patient welfare and maintain profitability for manufacturers.